De Novo designed 13 mer hairpin-peptide arrests insulin and inhibits its aggregation: role of OH–π interactions between water and hydrophobic amino acids†
Abstract
Background: Protein aggregation in the cellular systems can be highly fatal causing a series of diseases including neurodegenerative diseases like ALS, Alzheimer, Prion Diseases, Parkinson's and other diseases like type II diabetes. To date, there is no crucial mechanism invented that shows how a protein molecule unfolds or misfolds. Insulin fibrillation in type II diabetes is an alarming event that brings every year deaths of millions of people around the globe. Pharmaceutical companies are still in the cultivation of finding newer therapeutic agents which halt/impede insulin aggregation to combat diabetes II and improve the patient's life expectancy. Methods and Results: Here in this report, we have engineered four short 13 mer peptides (N-term-DMYY- N-term-DITT-
N-term-DITT- N-term-DIFF-
N-term-DIFF- N-term-KVYY-
N-term-KVYY- ) which target monomeric insulin in its globular form. The de Novo designed peptides are found to be non-cytotoxic in human HEK293 cells. Among these four peptides, only DITT-
) which target monomeric insulin in its globular form. The de Novo designed peptides are found to be non-cytotoxic in human HEK293 cells. Among these four peptides, only DITT-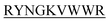 showed complete inhibition of insulin fibrillation, whereas DIFF-
showed complete inhibition of insulin fibrillation, whereas DIFF-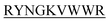 and DIYY-
and DIYY-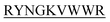 and KVYY-
and KVYY-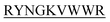 lost their functionality to impede insulin aggregation to a great extent. High-resolution multi-dimensional NMR experiments portrayed the 13 mer sequences of peptides in the beta-hairpin forms. A series of biophysical techniques like CD, ThT assay, DLS, SEM, ITC, size-exclusion chromatography, and molecular dynamics simulation strongly evidenced inhibition of insulin fibrillation by N-term-DITT-
lost their functionality to impede insulin aggregation to a great extent. High-resolution multi-dimensional NMR experiments portrayed the 13 mer sequences of peptides in the beta-hairpin forms. A series of biophysical techniques like CD, ThT assay, DLS, SEM, ITC, size-exclusion chromatography, and molecular dynamics simulation strongly evidenced inhibition of insulin fibrillation by N-term-DITT-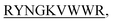 compared to those by the other peptides. Conclusion and significance: Here we tried to unravel how DITT-
compared to those by the other peptides. Conclusion and significance: Here we tried to unravel how DITT-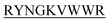 could impede insulin fibrillation.
could impede insulin fibrillation.



 Please wait while we load your content...
Please wait while we load your content...