Recent advancements in synthetic methodologies of 3-substituted phthalides and their application in the total synthesis of biologically active natural products
Abstract
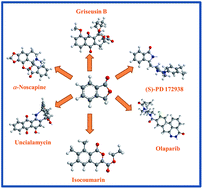
We have provided a critical review that focuses on key developments in the area of 3-substituted phthalides and their role in the development of important biologically active natural products. 3-Substituted phthalides are vital molecules owing to their fascinating biological activity. The scope, isolation, and characterization of various naturally occurring racemic and chiral 3-substituted phthalides have been covered. We have put significant emphasis on recently developed research methodologies for the synthesis of racemic and chiral 3-substituted phthalides. These newer approaches are essential for the development of newer and elegant strategies for the synthesis of phthalide-based or similar molecular architecture with broader substrate scope and higher stereoselectivities. Also, we have discussed the application of 3-substituted phthalides as a precursor for the synthesis of natural products and their analogs.
- This article is part of the themed collection: 2020 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...