6,N2-Diaryl-1,3,5-triazine-2,4-diamines: synthesis, antiproliferative activity and 3D-QSAR modeling†
Abstract
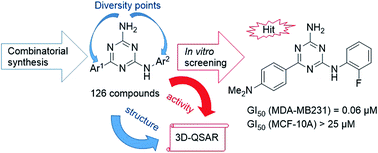
A library of 126 compounds with a 6,N2-diaryl-1,3,5-triazine-2,4-diamine scaffold was prepared using a one-pot, microwave-assisted method from readily available cyanoguanidine, aromatic aldehydes and arylamines. The three-component condensation of these reagents in the presence of hydrochloric acid was followed by the treatment with a base, which promoted a rearrangement of the dihydrotriazine ring and its dehydrogenative aromatization. The antiproliferative properties of the prepared compounds were evaluated using three breast cancer cell lines. The most promising results were obtained in the growth inhibition of the triple negative MDA-MB231 breast cancer cells. The active compounds were also selective against cancer cells and did not affect growth of the non-cancerous MCF-10A breast cell line. Analyzing the structure–activity relationship within the series, we built a 3D-QSAR model for the further design of more potent anticancer compounds.


 Please wait while we load your content...
Please wait while we load your content...