Mechanochemical approach to synthesize citric acid-soluble fertilizer of dittmarite (NH4MgPO4·H2O) from talc/NH4H2PO4 mixture†
Abstract
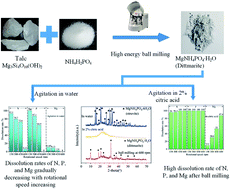
In this study, a citric acid-soluble fertilizer of dittmarite (NH4MgPO4·H2O) was synthesized by balling talc with NH4H2PO4. The effects of ball milling speed and milling time on the dissolution rates of N, P and Mg in deionized water and 2% citric acid were explored. Characterization technologies such as X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TG) and Scanning electron microscopy (SEM) were applied to test the prepared samples. In water, the prepared dittmarite was changed into struvite (NH4MgPO4·6H2O) with almost no N, P or Mg release, while the dissolution rates of nutrient elements reached almost 100% in 2% citric acid. The proposed work presented a facile and environmentally friendly method to produce CASF with high agricultural and ecological value.


 Please wait while we load your content...
Please wait while we load your content...