Synthesis of caffeic acid sulfonamide derivatives and their protective effect against H2O2 induced oxidative damage in A549 cells†
Abstract
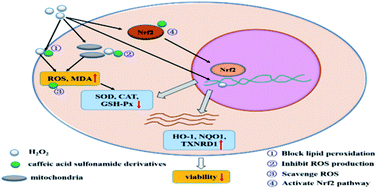
Exogenous antioxidants are considered as important therapeutic tools for oxidative stress associated disorders as they can regulate the redox state, which is associated with cell and organ function. Inspired by natural polyphenols, six new caffeic acid sulfonamide derivatives were synthesized by coupling sulfonamides to the backbone of caffeic acid with good yields. Their structure and lipophilicity were characterized by 1H nuclear magnetic resonance (NMR), 13C{1H} NMR, infrared spectroscopy (IR) and oil–water partition coefficient assay. Their free radical scavenging activity and antioxidant activity were assessed by DPPH assay and hydrogen peroxide (H2O2) induced oxidative stress in human lung carcinoma A549 cells. The oil–water partition coefficient results indicate that the conjugation of sulfonamides increases the lipophilicity of caffeic acid. The CASMD, CASDZ and CASN results show higher free radical scavenging effects compared with vitamin C. The derivatives do not show any inhibitory effect on the proliferation of A549 cells up to a concentration of 200 μM, except CASDZ which significantly inhibits the growth of A549 cells at a concentration of 200 μM. In addition, the obtained derivatives markedly attenuate H2O2 induced decrease of cell viability, inhibit the production of ROS and MDA, and promote the activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px). Besides, treatment of H2O2 stimulated A549 cells with caffeic acid sulfonamide derivatives further increases mRNA expression of NF-E2-related factor 2 (Nrf2) and its target genes, including heme oxygenase-1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1) and thioredoxin reductase 1 (TXNRD1). These results suggest that these new caffeic acid sulfonamide derivatives have higher lipophilicity and better antioxidant activities than the parent caffeic acid, and they might be able to control the antioxidant response in cells via the Nrf2 pathway.


 Please wait while we load your content...
Please wait while we load your content...