Synthesis of gemini ammonium sulfobetaine and its proppant suspension and gel-breaking mechanisms
Abstract
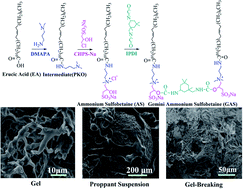
In this study, gemini ammonium sulfobetaine (GAS) is designed and synthesized using isophorone diisocyanate connecting the ammonium sulfobetaines (AS) to obtain a viscoelastic surfactant exhibiting better viscosification and salt resistance. AS is prepared using the monomers of erucic acid, N-dimethyl-1,3-propanediamine, and 3-chloro-2-hydroxypropanesulfonic acid sodium. The properties of GAS and its proppant suspension as well as the gel-breaking mechanisms are investigated. The critical micelle concentration of GAS is 2.1 × 10−7 mol mL−1. GAS exhibits good salt resistance, and the viscosity is considerably high under acidic conditions. At 0.5 Hz, the storage modulus G′ of GAS is 60, 120, and 640 mPa when the concentration is 0.3, 0.5, and 1.0 wt%, respectively. Its proppant suspension is optimal under acidic conditions. When the pH is high, the setting velocities are clearly observed to increase. When the pH is 12, the rate of decline is more than 50% after 200 min. Some of the worm-like micelles adsorbed on the proppant surface participate in the formation of the three-dimensional network, appropriately supporting the proppant-carrying performance. When potassium permanganate is used as the gel breaker, the characterization of the GAS gel-breaking liquid indicates that the double bond is disintegrrated by the gel breaker. Upon gel breaking, the average hydrodynamic radius of the GAS gel-breaking solution decreases to 176.2 nm from 492.3 nm.


 Please wait while we load your content...
Please wait while we load your content...