Temperature controlled condensation of nitriles: efficient and convenient synthesis of β-enaminonitriles, 4-aminopyrimidines and 4-amidinopyrimidines in one system†
Abstract
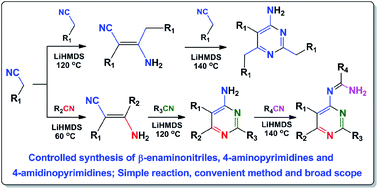
A series of β-enaminonitriles, 4-aminopyrimidines and 4-amidinopyrimidines were synthesized by condensation of organonitriles in one system. A wide variety of compounds were obtained in good to excellent yields by simply controlling the reaction temperature. The base-induced transformation process is easy for production. The scope and versatility of the method have been successfully demonstrated with 72 examples. The flexible and diversified characteristics of nitriles were introduced based on electronic effect, steric effect, position of substituted groups and intermolecular hydrogen bonding. An updated reaction mechanism is proposed based on the study of the stoichiometric reaction conditions at variable temperature, and on the investigation of products with cis–trans configuration transformation.


 Please wait while we load your content...
Please wait while we load your content...