Characteristics and hazards of the cinnamaldehyde oxidation process
Abstract
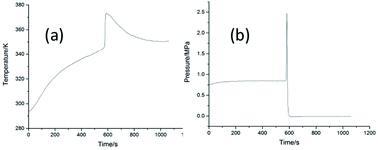
Pressure and temperature behavior of the cinnamaldehyde oxidation process was determined using a custom-designed mini closed pressure vessel test (MCPVT), which is a new method to investigate the stability and hazard assesment of the cinnamaldehyde oxidation reaction. The oxidation products were analyzed by gas chromatography-mass spectrometry (GC-MS). The results showed that cinnamaldehyde was stable under nitrogen atmosphere but very unstable under oxygen atmosphere. The initial oxidation products were analyzed by iodimetry and the cinnamaldehyde peroxide value could reach 139.44 mmol kg−1 when the oxidation temperature was 308 K. The oxidation kinetics of cinnamaldehyde were studied by using the pressure versus time (P–t) curves obtained from the MCPVT process. The reaction is a second-order reaction, the kinetic equation is ln k = −2233.66 × (1/T) + 11.19, and the activation energy Ea is 18.57 kJ mol−1 at 308–338 K. The explosion of the cinnamaldehyde oxidation reaction was observed by MCPVT, in which the onset temperature was 373 K. The main products of cinnamaldehyde oxidation are acetaldehyde, benzaldehyde, phenylacetaldehyde, acetophenone, 2-hydroxyphenyl acetone, cinnamaldehyde epoxide, benzoic acid, and cinnamic acid. Oxidation is a three-step process: (1) cinnamaldehyde reacts with oxygen to form peroxides; (2) complex oxidation reactions are caused by the thermal decomposition of peroxides; (3) rapid oxidation and thermal decomposition lead to explosion hazard.


 Please wait while we load your content...
Please wait while we load your content...