Unravelling the fibrillation mechanism of ovalbumin in the presence of mercury at its isoelectric pH†
Abstract
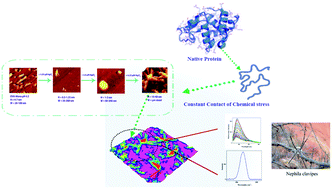
The intriguing resemblances of amyloid fibrils and spider silk in protein aggregation diseases have instigated the exploration of identical structural features if any in their oligomeric pathways. The serpin group protein, ovalbumin, on defolding in HgCl2 shares commonness to the micellar pathway of spidroins for their aggregation in response to a pH trigger. The structural feature changes from monomer to worm like fibril with a shift in the primary protein pH to slightly acidic pH (4.5), and then proceeds through a secondary nucleation pathway to ‘hillock’ and ‘hydra’ like protofibrils rich in β-sheet and random coil conformers upon exposure to mercury. The findings are backed by atomic force microscopy, confocal Raman spectroscopy and fluorescence measurements. Unlocking such structural features can favorably assist in the design of therapeutics.

 Please wait while we load your content...
Please wait while we load your content...