Preparation of cyclic imides from alkene-tethered amides: application of homogeneous Cu(ii) catalytic systems†
Abstract
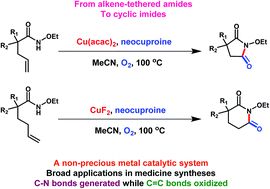
A Cu-based homogeneous catalytic system was proposed for the preparation of imides from alkene-tethered amides. Here, O2 acted as a terminal oxidant and a cheap and easily available oxygen source. The cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and the formation of C–N bonds were catalyzed by Cu(II) salts with proper nitrogen-containing ligands under 100 °C. The synthesis approach has potential applications in pharmaceutical syntheses. Moreover, scaled-up experiments confirmed the practical applicability.
C bonds and the formation of C–N bonds were catalyzed by Cu(II) salts with proper nitrogen-containing ligands under 100 °C. The synthesis approach has potential applications in pharmaceutical syntheses. Moreover, scaled-up experiments confirmed the practical applicability.


 Please wait while we load your content...
Please wait while we load your content...