High capacity rock salt type Li2MnO3−δ thin film battery electrodes
Abstract
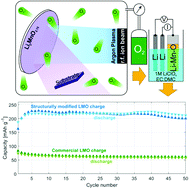
Recent investigations of layered, rock salt and spinel-type manganese oxides in composite powder electrodes revealed the mutual stabilization of the Li–Mn–O compounds during electrochemical cycling. A novel approach of depositing such complex compounds as an active cathode material in thin-film battery electrodes is demonstrated in this work. It shows the maximum capacity of 226 mA h g−1 which is superior in comparison to that of commercial LiMn2O4 powder as well as thin films. Reactive ion beam sputtering is used to deposit films of a Li2MnO3−δ composition. The method allows for tailoring of the active layer's crystal structure by controlling the oxygen partial pressure during deposition. Electron diffractometry reveals the presence of layered monoclinic and defect rock salt structures, the former transforms during cycling and results in thin films with extraordinary electrochemical properties. X-ray photoelectron spectroscopy shows that a large amount of disorder on the cation sub-lattices has been incorporated in the structure, which is beneficial for lithium migration and cycle stability.


 Please wait while we load your content...
Please wait while we load your content...