Crystallization of colourless hexanitratoneptunate(iv) with anhydrous H+ countercations trapped in a hydrogen bonded polymer with diamide linkers†
Abstract
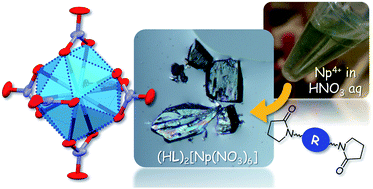
Colourless crystalline compounds of centrosymmetric [Np(NO3)6]2− were yielded from 3 M HNO3 aq in the presence of double-headed 2-pyrrolidone derivatives (L). In the obtained crystal structures, H+ was also involved as a countercation to compensate for the negative charge of [Np(NO3)6]2−, where the initial hydration around H+ was fully removed during crystallization despite it having the strongest hydration enthalpy. Instead, this anhydrous H+ was captured by L to form a [H+⋯L]n hydrogen bonded polymer. In [Np(NO3)6]2−, the Np4+ centre is twelve-coordinated with 6 bidentate NO3−, and therefore, present in an icosahedral geometry bearing inversion centre. In such a centrosymmetric system, any f–f transitions stemming from the 5f3 electronic configuration of Np4+ are electric-dipole forbidden. This is the reason why the compounds currently obtained were colourless unlike ordinary Np(IV) species, which are olive-green.
- This article is part of the themed collection: Shining a Light on the f-Block


 Please wait while we load your content...
Please wait while we load your content...