Synthesis of the pentasaccharide repeating unit of the O-antigen from Enterobacter cloacae C4115 containing the rare α-d-FucNAc†
Abstract
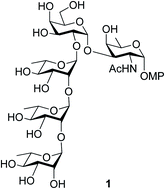
Total synthesis of the pentasaccharide repeating unit associated with the O-antigen of Enterobacter cloacae C4115 is reported. The synthesis of the said oligosaccharide was accomplished through rational protecting group manipulations on commercially available monosaccharides followed by stereoselective glycosylations either by activation of thioglycosides or glycosyl trichloroacetimidates and was found to be productive. Towards the synthesis of the rare sugar unit, α-D-FucNAc in this case, it was established that the methoxymethyl (MOM) group is advantageous over the earlier reported tetrahydro pyran (THP) protection. The effect of MOM-protection was successfully tested for the synthesis of a rare sugar synthon which can serve as a precursor to the rare D-fucosamine residue.


 Please wait while we load your content...
Please wait while we load your content...