Synthesis of new N,N′-Pd(Pt) complexes based on sulfanyl pyrazoles, and investigation of their in vitro anticancer activity†
Abstract
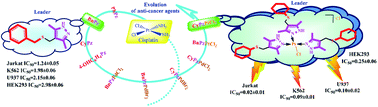
The synthesis of new N,N′-mononuclear bi-ligand Pd(II) and tri-ligand Pt(II)complexes bearing sulfanyl(phenyl, benzyl, cyclohexyl, 4-hydroxyphenyl)3,5-dimethyl-1H-pyrazole ligands has been carried out. The obtained compounds were studied for apoptosis-inducing activity and effect on the cell cycle for Jurkat, K562, and U937 neoplastic cell cultures and conditionally normal human embryonic kidney HEK293 cells. The cells showed the highest sensitivity to platinum and palladium complexes in comparison with ligands and cisplatin. The cytotoxic properties are enhanced for compounds with cyclohexyl substituents at the S-atom in sulfanyl pyrazoles and complexes.


 Please wait while we load your content...
Please wait while we load your content...