Unravelling the role of temperature in a redox supercapacitor composed of multifarious nanoporous carbon@hydroquinone†
Abstract
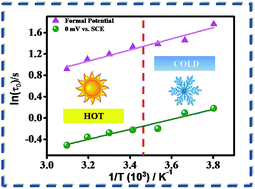
The intermittency of renewable energy sources has led to the invention of supercapacitors. The variation of temperature impacts their working capability particularly in the regions which are too hot or too cold. Herein, the effect of temperature on the double layer formation and the redox mechanism of hydroquinone adsorbed on multifarious nanoporous carbon (MNC) have been reported. The studies have been carried out in 2 M H2SO4 electrolyte solution within a temperature range of −10 to 50 °C. The maximum specific capacitance of the composite material drops from 319 F g−1 at 50 °C to 213 F g−1 at −10 °C. An equivalent circuit model has been chosen to fit the EIS spectra at the double layer potential and formal potential. Subsequently, an Arrhenius type plot has been constructed to calculate the activation energy of the system which revealed 8.69 and 7.77 kJ mol−1 activation energy at the formal potential and double layer potential respectively. The composite also shows excellent cyclability even at enhanced temperatures, which is a major requirement for the application of these supercapacitors in vehicles and other electrical equipment.


 Please wait while we load your content...
Please wait while we load your content...