High-pressure formation of antimony nitrides: a first-principles study†
Abstract
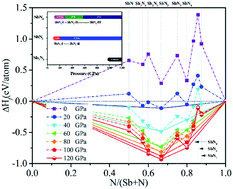
The structural phase transition, electronic properties, and bonding properties of antimony nitrides have been studied by using the first principles projector augmented wave method. The relationship between the formation enthalpy and the composition of the Sb–N system has been explored. The novel Sb2N3 with the Cmcm space group is stable in a narrow pressure range from 100 GPa to 120 GPa. Apart from the Sb2N3, two nitrogen-rich phases SbN2 and SbN4 were predicted. The SbN2 with the C2/m space group is stable at 12 GPa and then transforms to the high-pressure phase at 23 GPa. The nitrogen-rich SbN4 appears at 14 GPa then undergoes C2/m → P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) → P
→ P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) phase transitions, and the calculated pressures of the phase transitions are 31 and 60 GPa, respectively. The nitrogen-rich SbN2 and SbN4 have similar structural features. Both SbN2 and SbN4 can be seen as a sandwich structure composed of the Sb–N layers and N2 dimers. The pressure-induced phase transitions of SbN2 and SbN4 are accompanied by the electron transfer between the Sb–N layers and N2 dimers. Moreover, the nitrogen-rich SbN4 has a higher energy density of 2.42 kJ g−1 and is a potentially high energy density material.
phase transitions, and the calculated pressures of the phase transitions are 31 and 60 GPa, respectively. The nitrogen-rich SbN2 and SbN4 have similar structural features. Both SbN2 and SbN4 can be seen as a sandwich structure composed of the Sb–N layers and N2 dimers. The pressure-induced phase transitions of SbN2 and SbN4 are accompanied by the electron transfer between the Sb–N layers and N2 dimers. Moreover, the nitrogen-rich SbN4 has a higher energy density of 2.42 kJ g−1 and is a potentially high energy density material.


 Please wait while we load your content...
Please wait while we load your content...