Aggregation-induced emission compounds based on 9,10-diheteroarylanthracene and their applications in cell imaging†
Abstract
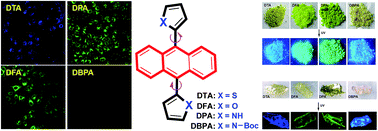
Four centrosymmetric 9,10-diheteroarylanthracene (DHA) derivatives, including 9,10-dithienylanthracene (DTA), 9,10-difurylanthracene (DFA), 9,10-di-(N-t-butyloxycarboryl-2-pyrryl)anthracene (DBPA), and 9,10-dipyrrylanthracene (DPA) have been synthesized and characterized. All of these DHA derivatives displayed distinct aggregation-induced emission (AIE) behaviors except for DBPA, which showed typical aggregation-caused quenching (ACQ) properties. Their crystal structures exhibited nonplanar conformations on account of the intramolecular torsional effects and intramolecular interactions in rigid molecules. The investigation of the effects of the anthracene core and the side heterocyclic units on the AIE properties demonstrated that the heterocycle moiety is the key factor for the AIE features. These DHA AIEgens exhibited excellent bioimaging performance under physiological conditions.


 Please wait while we load your content...
Please wait while we load your content...