Synthesis of phosphonoacetate analogues of the second messenger adenosine 5′-diphosphate ribose (ADPR)†
Abstract
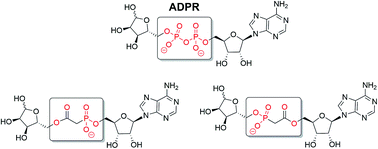
Adenosine 5′-diphosphate ribose (ADPR) is an intracellular signalling molecule generated from nicotinamide adenine dinucleotide (NAD+). Synthetic ADPR analogues can shed light on the mechanism of activation of ADPR targets and their downstream effects. Such chemical biology studies, however, are often challenging due to the negatively charged pyrophosphate that is also sensitive to cellular pyrophosphatases. Prior work on an initial ADPR target, the transient receptor potential cation channel TRPM2, showed complete pyrophosphate group replacement to be a step too far in maintaining biological activity. Thus, we designed ADPR analogues with just one of the negatively charged phosphate groups removed, by employing a phosphonoacetate linker. Synthesis of two novel phosphonoacetate ADPR analogues is described via tandem N,N′-dicyclohexylcarbodiimide coupling to phosphonoacetic acid. Neither analogue, however, showed significant agonist or antagonist activity towards TRPM2, underlining the importance of a complete pyrophosphate motif in activation of this particular receptor.


 Please wait while we load your content...
Please wait while we load your content...