Theoretical evaluation of inter-ion and intra-ion isotope effects in fragmentation: insights into chlorine and bromine isotope effects of halogenated organic compounds occurring in electron ionization mass spectrometry†
Abstract
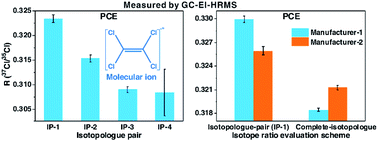
Revelation of chlorine and bromine isotope effects in fragmentation is crucial for compound-specific isotope analysis of chlorine/bromine (CSIA-Cl/Br) using gas chromatography-electron ionization mass spectrometry (GC-EI-MS), but theoretical fundamentals of the isotope effects remain unclear. Herein, this study provides a theoretical basis for elucidating the details and implications of chlorine and bromine isotope effects occurring in dehalogenation reactions in EI-MS. Inter-ion and intra-ion isotope effects can occur in dehalogenation reactions in EI-MS, and affect chlorine/bromine isotope ratios. In a dehalogenation reaction, inter-ion isotope effects increase the isotope ratio of a precursor ion but decrease that of its product ion. On the other hand, intra-ion isotope effects can only affect (increase) the isotope ratio of a product ion, and have no effect on its precursor ion. The chlorine/bromine isotopologue distributions of ions measured by EI-MS are deduced to be non-binomial (nonrandom), regardless of the initial isotopologue distributions prior to fragmentation. The bulk chlorine/bromine isotope ratio of an ion cannot be exactly achieved with a calculation scheme using an arbitrary pair or pairs of neighboring chlorine/bromine isotopologues, but can be calculated with complete isotopologues of the ion. The isotope ratio of a compound calculated with a pair/pairs of neighboring isotopologues could not accurately reflect the trueness, even though it has been calibrated with external isotopic standard(s), due to different isotopologue distributions of the analyte and external isotopic standard(s). The conclusions derived from theoretical derivation have been experimentally proven with the isotopically distinct standards of tetrachloroethylene and trichloroethylene from different manufacturers. The findings of this study are conducive to CSIA-Cl/Br using GC-EI-MS to obtain high-quality data, and provide new insights into the actual chlorine/bromine isotopologue distributions of chlorinated/brominated organic compounds.


 Please wait while we load your content...
Please wait while we load your content...