Synthesis of cucurbitacin IIa derivatives with apoptosis-inducing capabilities in human cancer cells†
Abstract
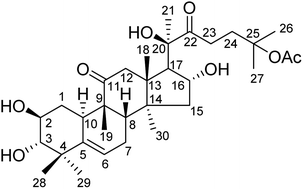
Twenty-one cucurbitacin IIa derivatives were synthesized and screened for cytotoxic activity. Their structures were established using 1H NMR, 13C NMR, and LC-MS spectroscopic data. The absolute configuration of the derivatives was determined by single crystal diffraction. In sulforhodamine B (SRB) assays, nearly all compounds displayed low cytotoxicity toward normal human cells (HEK293). However, some derivatives displayed high cytotoxicity, in the low μM range, toward several human tumor cell lines (SKOV3, HT29, HEPG2, MCF-7, and LOVO). Low IC50 values were obtained, especially for acetyl-protected product 2, 2,4,6-trichlorophenylhydrazine derivative 4a, and 2-hydrazinopyridine derivative 4d. In particular, compounds 2 and 4d showed low IC50 values of 1.2 ± 0.01 and 2.2 ± 0.19 μM against SKOV3 cells. These compounds were submitted to extensive biological testing, which showed that compounds 2 and 4a did not inhibit tumor cells by influencing the cell cycle. Furthermore, compound 4a triggered the apoptotic pathway in cancer cells, showing high apoptosis ratios. This study mainly changed the structure of cucurbitacin tetracyclic triterpenoids and provided a novel tetracyclic skeleton derived from natural products that provided further references for the future modification of cucurbitacin tetracyclic triterpenoids.


 Please wait while we load your content...
Please wait while we load your content...