Unraveling how the Gly526Ser mutation arrests prostaglandin formation from arachidonic acid catalyzed by cyclooxygenase-2: a combined molecular dynamics and QM/MM study†
Abstract
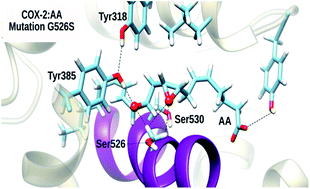
Cyclooxygenases (COXs) are the enzymes responsible for the biosynthesis of prostaglandins, eicosanoids that play a major role in many physiological processes. Particularly, prostaglandins are known to trigger inflammation, and COX-2, the enzyme isoform associated with this inflammatory response, catalyzes the cyclooxidation of arachidonic acid, leading to prostaglandin G2. For this reason, COX-2 has been a very important pharmacological target for several decades now. The catalytic mechanism of COX-2, a so-called all-radical mechanism, consists of six chemical steps. One of the most intriguing aspects of this mechanism is how COX-2 manages to control the regio- and stereospecificity of the products formed at each step. Mutagenesis experiments have previously been performed in an attempt to find those hot-spot residues that make such control possible. In this context, it is worth mentioning that in experiments with the Gly526Ser COX-2 mutant, prostaglandins were not detected. In this paper, we have combined molecular dynamics simulations and quantum mechanics/molecular mechanics calculations to analyze how the COX-2 catalytic mechanism is modified in the Gly526Ser mutant. Therefore, this study provides new insights into the COX-2 catalytic function.


 Please wait while we load your content...
Please wait while we load your content...