Pre-clinical pharmacokinetic and pharmacodynamic modelling study of 4-hydroxyisoleucine using validated ultra-performance liquid chromatography-tandem mass spectrometry†
Abstract
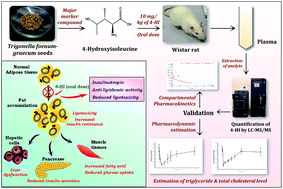
A reliable and sensitive ultra-performance liquid chromatography-tandem mass spectrometry-based method has been developed for the estimation of 4-hydroxyisoleucine (4-HI), a potent insulinotropic and hypolipidemic agent. The extraction of 4-HI from plasma was accomplished by the protein precipitation technique using L-isoleucine as an internal standard. The separation of analytes was achieved with a mobile phase consisting of acetonitrile and 0.1% formic acid in an isocratic flow system on a BEH Shield RP-18 column (150 mm × 2.1 mm, 1.7 μm). 4-HI and L-isoleucine were detected using an electrospray ionization (ESI) ion source, using multiple reaction monitoring (MRM) in positive ion mode. The precursor to product ion transitions of 4-HI and L-isoleucine were found at m/z values of 148.19 > 74.02 and 132.17 > 69.04, respectively. As per the guidelines for bioanalytical methods, all validation parameter results were within the acceptable range. The method exhibited a robust and reproducible linearity range of 1–5000 ng mL−1 with a coefficient of regression of 0.9999. The method was successfully applied for the estimation of pharmacokinetic parameters after oral administration of 4-HI (10 mg kg−1) in Wistar rats, by using Thoth Pro (version: 4.3) software. Herein, the two-compartment model was statistically fitted based on AIC and SBC values for evaluation of the pharmacokinetic parameters of 4-HI. Pharmacodynamic studies were also performed by measuring the levels of triglyceride and total cholesterol, and showed that the pharmacokinetic and pharmacodynamic data of 4-HI correlated with each other.


 Please wait while we load your content...
Please wait while we load your content...