Biotransformation of α-terpineol by Alternaria alternata†
Abstract
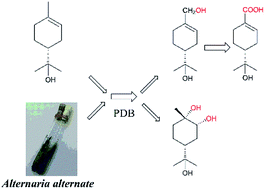
α-Terpineol (1), the main volatile constituent in some traditional Chinese medicines, has been reported to be metabolized to 4R-oleuropeic acid by the larvae of common cutworms. The present study verified that α-terpineol could be converted to 4R-oleuropeic acid (2) and (1S,2R,4R)-p-menthane-1,2,8-triol (3) by Alternaria alternata fermentation. Using shortened fermentation times, 7-hydroxy-α-terpineol (2a) was identified as an oxidative intermediate, which was consistent with the hypothesis put forward by previous studies. Cytochrome P450 enzymes were also confirmed to catalyze this biotransformation. This is the first study on the biotransformation of α-terpineol by microbial fermentation.


 Please wait while we load your content...
Please wait while we load your content...