Visible-light-induced selectivity controllable synthesis of diamine or imidazoline derivatives by multicomponent decarboxylative radical coupling reactions†
Abstract
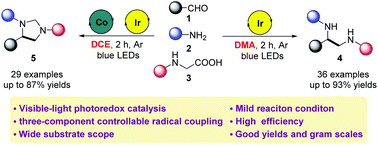
A visible-light-induced and photoredox-catalyzed three-component selectivity controllable radical reaction of the readily available carbonyl compounds, amines and glycine derivatives has been developed. The nature of the products could be successfully controlled by varying the reaction solvent and oxidant, and various vicinal diamine or imidazoline derivatives could be afforded selectively in moderate to excellent yields. A series of experimental studies were carried out to gain insight into the reaction and a plausible reaction mechanism was proposed. In addition, this protocol was successfully applied in the construction of the mianserine skeleton.


 Please wait while we load your content...
Please wait while we load your content...