Angular ladder-type meta-phenylenes: synthesis and electronic structural analysis†
Abstract
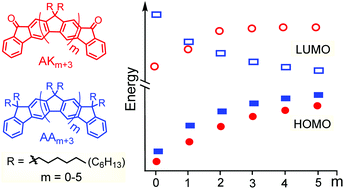
Herein, we report the synthesis of two new series of angular (all-syn) ladder-type meta-[n]phenylenes (LMP, n = 3–8). One series contains keto groups at the termini bridges, denoted angular keto (AKn), the second contains alkyl groups at all bridge sp3 carbons, denoted angular alkyl (AAn). Their electronic and structural properties were delineated by a combination of electrochemistry and spectroscopic (UV-Vis and emission) methods and further supported by DFT calculations. Interestingly, experimental and DFT data show that changing the bridging group at the termini from alkyl (AAn) to keto (AKn) gives an increase in the first reduction potentials and LUMO energies, as the π-system is extended. Also, the charge (de)localization behavior is different for these two species; while the AAn compounds stablize charge with Robin-Day class III, the AKn compounds show a clear switch from class III to class II. In comparison with the linear analogues (LKn and LAn), DFT results reveal a shape independency of the charge (de)localization mechanism in acceptor–π-acceptor series (AKn/LKn).


 Please wait while we load your content...
Please wait while we load your content...