Ruthenium(ii)-catalyzed acyloxylation of the ortho-C–H bond in 2-aroyl-imidazoles with carboxylic acids†‡
Abstract
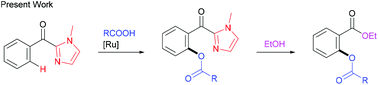
The reaction of 2-aroyl-imidazoles with carboxylic acids using [RuCl2(p-cymene)]2 as the catalyst and Ag2CO3 as the oxidant results in ortho-C–H acyloxylation to afford acyloxylation products, in which the imidazole group functions as a directing group. A wide range of functional groups are tolerated in the reaction. The directing group can be easily converted to the corresponding esters under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...