Metal-free reductive coupling of aliphatic aldehydes/ketones with 4-cyanopyridines: expanded scope and mechanistic studies†
Abstract
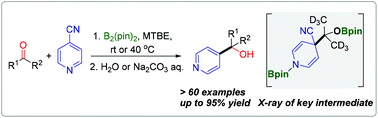
An efficient and practical bis(pinacolato)diboron (B2pin2) mediated radical–radical cross-coupling of 4-cyanopyridine with aliphatic aldehydes/ketones has been established. The metal-free protocol features simple and mild reaction conditions and excellent functional group tolerance, and provides a general and convenient route to construct a wide range of pyridine-functionalized alcohols without transition-metal or organometallic reagents. The mechanistic studies offer significant insights into the key radical–radical coupling process between the pyridine-boryl radical and the ketyl radical, and the key radical cross-coupling intermediate was structurally characterized by NMR spectroscopy and X-ray diffraction, which is consistent with density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...