External-photocatalyst-free visible-light-mediated aerobic oxidation and 1,4-bisfunctionalization of N-alkyl isoquinolinium salts†
Abstract
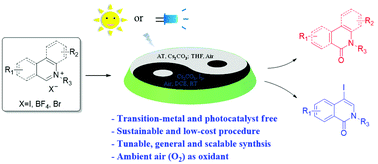
Visible-light-mediated aerobic alternate transformations of N-alkyl isoquinolinium/quinolinium salts in the absence of any extra added photocatalyst are reported. Here, a highly concise, sustainable and low-cost protocol to produce unsaturated lactams from oxidation of their corresponding isoquinolinium or quinolinium salts using air as an oxidant was developed. As important building blocks for further modification of biologically active isoquinolones, 4-iodoisoquinolinones were also produced through 1,4-bisfunctionalization of isoquinolinium salts without the use of any transition metal. Gram-scale synthesis was achieved under sunlight with exposure to the open air. Mechanism studies suggested that singlet oxygen generation triggered by isoquinolinium/quinolinium salts under visible light irradiation and trace water may lead to multiple pathways for the aerobic transformations of N-alkyl iminium salts.


 Please wait while we load your content...
Please wait while we load your content...