Hydride transfer enabled switchable dearomatization of indoles in the carbocyclic ring and the pyrrole ring†
Abstract
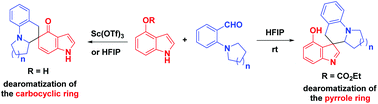
Hydride transfer enabled the first success of the regioselective dearomatization of indoles in the carbocyclic ring and the pyrrole ring, which was induced by ortho-quinone methides and vinylogous iminium intermediates. A variety of spiro cyclohexaketenes featuring the dearomatized indoles and carbazoles as well as spiroindolenines were provided in moderate to high yields. The formidable challenge of switchable dearomatization of fused bicyclic aromatic compounds was addressed.


 Please wait while we load your content...
Please wait while we load your content...