Copper-catalyzed asymmetric tandem borylative addition and aldol cyclization†
Abstract
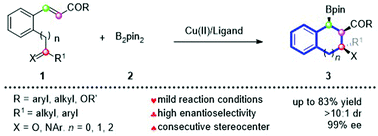
A highly enantioselective asymmetric copper-catalyzed tandem conjugate addition/aldol cyclization of electron-deficient olefins with B2pin2 was developed, which provided a rapid access to multi-substituted indanes bearing three consecutive chiral stereogenic centers at the same side of the indane ring. The features of this reaction include high chemo-, diastereo- and enantio-selectivity (up to 99% ee) and mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...