An approach towards the construction of the tetracyclic skeleton of palhinine alkaloids†
Abstract
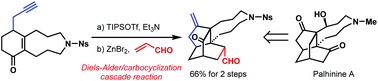
A concise synthesis of the tetracyclic skeleton of palhinine alkaloids is described. The approach features a bifunctional Lewis acid promoted Diels–Alder/carbocyclization cascade for the rapid construction of the isotwistane (tricyclo[4.3.1.03,7]decane) core. This key reaction is highly diastereoselective and could be carried out on gram scale. The cyclization product already possesses the completed ring system of palhinine alkaloids and could potentially be further functionalized to palhinine A.


 Please wait while we load your content...
Please wait while we load your content...