Oxidative cascade cyclization of 2-cyano-3-arylaniline derived acrylamides with toluenes, ethers, aliphatic alcohols or simple alkanes†
Abstract
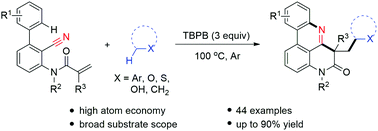
An oxidative radical cascade cyclization of 2-cyano-3-arylaniline derived acrylamides with hydrocarbons is described. The present reaction exhibits a wide substrate scope, and toluenes, ethers, aliphatic alcohols and simple alkanes can be employed as coupling partners, providing access to alkyl substituted pyrido[4,3,2-gh]phenanthridines.
- This article is part of the themed collection: 2020 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...