Transition metal-free synthesis of α-aryl ketones via oxyallyl cation capture with arylboronic acids†
Abstract
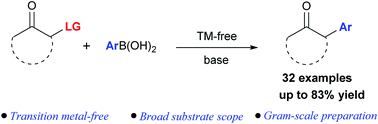
Herein, we describe a novel transition metal-free, umpolung strategy for the α-arylation of ketones by capturing transient oxyallyl cations with arylboronic acids. A variety of α-arylated ketones were obtained in moderate to good yields with broad substrate tolerance under simple reaction conditions. This process provides a complementary strategy compared with traditional transition metal-catalyzed methods.


 Please wait while we load your content...
Please wait while we load your content...