Efficiently diastereoselective synthesis of functionalized hydro-carbazoles by base-mediated tandem annulation of 1-(2-amino-aryl)prop-2-en-1-ones and sulfur ylide†
Abstract
The base-promoted [3 + 3]/[1 + 4] tandem reaction of tosyl-protected o-amino α,β-unsaturated ketones and crotonate-derived sulfur ylide for the efficiently diastereoselective synthesis of functionalized hydrocarbazoles is reported. The reaction proceeded smoothly and gave the desired products in high yields and excellent diastereoselectivities under mild conditions. The hydrocarbazoles could be readily transformed into functionalized carbazoles.
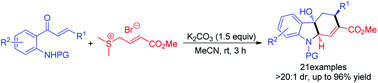


 Please wait while we load your content...
Please wait while we load your content...