Pd/light-induced alkyl–alkenyl coupling reaction between unactivated alkyl iodides and alkenylboronic acids†
Abstract
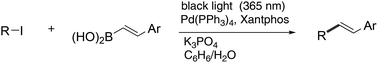
Alkyl–alkenyl coupling reaction between unactivated alkyl iodides and 2-arylalkenylboronic acids utilizing a Pd/light combined system was studied. This reaction provided 2-alkylarylalkenes in good yields. It is proposed that in this reaction alkyl radicals are formed by SET from a Pd catalyst to alkyl iodides, which then undergo addition reactions to form C–C double bonds of alkenyl boronic acids and the subsequent β-fragmentation of boronyl radicals leads to the formation of alkyl-substituted arylalkenes.


 Please wait while we load your content...
Please wait while we load your content...