NHC-catalyzed β-specific addition of N-based nucleophiles to allenoates†
Abstract
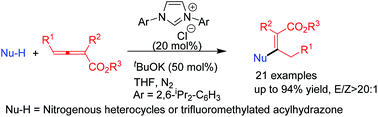
N-Heterocyclic carbene (NHC) catalyzed regiospecific β-additions of nitrogen-containing nucleophiles including nitrogenous heterocycles and trifluoromethylated acylhydrazone to allenoates were accomplished under mild reaction conditions, harnessing NHC as a Lewis base to furnish branched N-alkyl compounds in moderate to good yields with high E/Z selectivity and broad substrate scope. DFT calculations on the possible reaction mechanisms show that the β-addition pathway is more energetically favourable than the γ-addition pathway, which is in agreement with the experimental observations.


 Please wait while we load your content...
Please wait while we load your content...