Transition-metal-free aerobic C–O bond formation via C–N bond cleavage†
Abstract
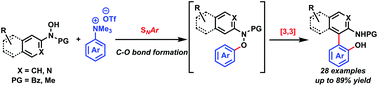
Herein, we disclosed a transition metal-free cascade protocol for the construction of 2-hydroxy-2′-amino-1,1′-biaryls that are difficult to prepare by employing conventional methods. The nucleophilic substitution of arylhydroxylamines to arylammonium salts at room temperature generates transient N,O-diarylhydroxylamines that could rapidly undergo a tandem process, including a [3,3]-sigmatropic rearrangement and rearomatization to afford NOBIN analogues.
- This article is part of the themed collection: 2020 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...