Aromaticity and tautomerism of a 4n π electron dihydrohexaazapentacene†
Abstract
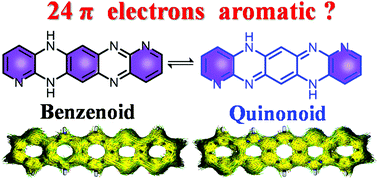
Tautomerism is an interesting phenomenon that allows different properties to coexist in the same molecule. However, the understanding of tautomerism of large antiaromatic skeletons is very limited. We report herein the intriguing tautomerism behaviors of a new hexazapentacene derivative, named DHHAP. In solution, DHHAP exists as a mixture of benzenoid and quinonoid tautomers in a rough ratio of 1 : 1. DFT calulations reveal that DHHAP is slightly more stable than its 4n + 2π hexazapentacene counterpart although it has 4n π electrons. DHHAP exhibits different halochromic behaviors upon addition of strong and mild acids. Despite multiple protonation possibilities, careful NMR investigation revealed that only one di-protonation process occurs at symmetrical sites on the quinonoid structure, which is attributed to electrostatic potential-induced protonation regioselectivity. Femtosecond transient absorption (fs-TA) spectroscopy revealed different excited state dynamics of DHHAP-related species. Deep understanding of the tautomerism and aromaticity of dihydrohexazapentacene may pave a new way for the design of stable 4n π heteroacenes towards novel organic semiconductors and sensing materials.


 Please wait while we load your content...
Please wait while we load your content...