Synthesis of α-amino nitriles through one-pot selective Ru-photocatalyzed oxidative cyanation of amines†‡
Abstract
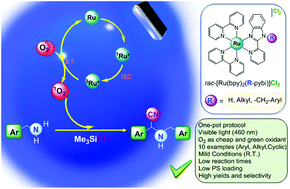
Photocatalysis is an expanding tool designed to synthesize organic molecules in a very efficient and selective way, using mild and environmentally friendly conditions. Thus, the development of new photosensitizers and photocatalytic protocols can further boost the applicability of photocatalysis in preparative chemistry. In this paper, we have conceived a family of Ru(II) polypyridyl complexes of general formula [Ru(bpy)2(N^N)]Cl2 (bpy = 2,2′-bipyridine) bearing the 2-pyridylbenzimidazole (Hpybim) scaffold in the ancillary ligands (N^N = R-pybim). Moreover, using these Ru(II) derivatives as photosensitizers we have described a protocol for the synthesis of imines from primary and secondary amines and also a simple, efficient and selective methodology for the one-pot preparation of α-amino nitriles from primary and secondary amines involving the consecutive photooxidation of the amine substrate and the cyanation of the imine intermediate. In addition, we have formulated a mechanism for the afore-mentioned transformations based on the participation of singlet oxygen.


 Please wait while we load your content...
Please wait while we load your content...