π-Type halogen bonding enhanced the long-lasting room temperature phosphorescence of Zn(ii) coordination polymers for photoelectron response applications†
Abstract
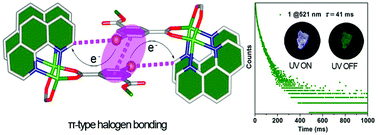
The development of non-noble metal ions with potential organic phosphor ligands through strong coordination bonds can afford an effective platform for achieving persistent and tunable phosphorescence at room temperature. However, successful examples of coordination polymers (CPs) or metal–organic frameworks (MOFs) exhibiting long-lasting phosphorescence emission are still rarely explored. In this work, a new strategy has been used for the first time to achieve long-lasting phosphorescence via π-type halogen bonding in coordination polymers. By the selection of two isomers 5-BIPA and 4-BIPA with different Br atom substitution positions in isophthalic acid (IPA), two new Zn(II)-based coordination polymers exhibiting long-lasting phosphorescence emission up to the order of magnitude of millisecond (ms) were presented, which were further revealed by theoretical calculations. Optoelectronic measurements indicated that 1 showed a high photoelectron response because the delocalized π-type halogen bonding and long range order of π-conjugated chains afforded large electron channels for efficient charge transport.


 Please wait while we load your content...
Please wait while we load your content...