Ligand substitution induced single-crystal-to-single-crystal transformations in two Ni(ii) coordination compounds displaying consequential changes in proton conductivity†
Abstract
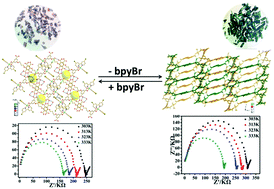
The discrete mononuclear [Ni(H2L)(bpyBr)2]·2H2O (1) and one-dimensional [Ni2(H2L)2(bpyBr)2(H2O)3]n (2) coordination compounds were obtained and characterized (H4L = 5,5′-(butane-1,4-diylbis(oxy))diisophthalic acid; bpyBr = 4,4′-dibromo-2,2′-bipyridyl). The two compounds can undergo SC–SC transformation into each other in a process stimulated by excess H4L or bpyBr that involves coordination bond cleavage and formation, which is a very rare case and should be defined as an SN2 nucleophilic ligand substitution reaction of the coordination compounds. Additionally, the proton conductivities of the Nafion membrane doped by compounds 1 and 2 were studied. Compounds 1 and 2 can enhance the proton conductivity of the composite membrane to about 51.80% and 20.94% higher than that of pure Nafion. It is speculated that the high proton density offered by the uncoordinated protonated carboxylate groups may endow the two compounds with good conductivities. In addition, the better hydrophilicity and stronger acidity of compound 1 deduced from the structure analyses and verified by the water uptake tests and IR spectra may lead to its higher conductivity.


 Please wait while we load your content...
Please wait while we load your content...