Lysosome-targeted iridium(iii) compounds with pyridine-triphenylamine Schiff base ligands: syntheses, antitumor applications and mechanisms†
Abstract
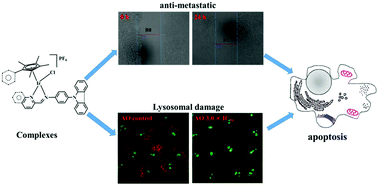
Six N-phenylcarbazole/triphenylamine modified half-sandwiched iridium(III) Schiff base compounds ([(η5-Cpx)Ir(N^N)Cl]PF6) were synthesised and characterised in this study. The regulation and introduction of Schiff bases increased the antitumor activity of these compounds (IC50: 1.4 ± 0.1 μM–11.5 ± 0.5 μM). The highest antitumor activity exhibited by these compounds was nearly 13 times that of clinical cisplatin. Interestingly, these compounds could also effectively block the migration of cancer cells. These compounds were observed to bind to proteins (binding constant: ∼104 M−1) and transport through serum protein, catalyse the oxidation of the coenzyme nicotinamide-adenine dinucleotide, and increase reactive oxygen species levels in cells, which resulted in an antitumor mechanism of oxidation. Laser confocal microscopy and flow cytometry studies confirmed that these compounds possessed an energy-dependent cellular uptake mechanism, effectively accumulated in lysosomes (Pearson co-localization coefficient: ∼0.75), damaged the integrity of acidic lysosomes, disrupted the cell cycle, induced a change in mitochondrial membrane potential, and eventually led to apoptosis. All these findings suggest that these compounds are potential antitumor agents with dual functions: metastasis inhibition and lysosomal damage.


 Please wait while we load your content...
Please wait while we load your content...