Azopyridine: a smart photo- and chemo-responsive substituent for polymers and supramolecular assemblies†
Abstract
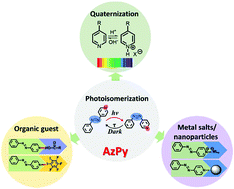
Azo dyes, such as azobenzene, are able to convert absorbed light into motion or deformation on the macroscopic scale on the basis of their remarkable ability to undergo repeatedly and in 100% yield reversible trans-to-cis photoisomerization. Current needs for multiresponsive and fast photoswitches have led to the development of heteroaryl azo dyes, such as azopyridine. This remarkable azo compound combines the photoresponse of the azo chromophore with the chemistry of the pyridine ring, in particular its responsiveness to changes in pH and its ability to form hydrogen- and halogen-bonds. This mini-review summarizes key features of the photoisomerization of polymer-tethered azopyridine in aqueous media and describes a few recent research accomplishments in emerging areas that have benefited of the fast thermal cis-to-trans relaxation characteristics of azopyridinium or H-bonded azopyridine. It also discusses the effects of the photoisomerization of azopyridine on the thermoresponsive properties of azopyridine-tethered heat-sensitive polymers. Overall, azopyridine is a highly versatile actuator to consider when designing photo/multiresponsive polymeric materials.


 Please wait while we load your content...
Please wait while we load your content...