An organocatalytic ring-opening polymerization approach to highly alternating copolymers of lactic acid and glycolic acid†
Abstract
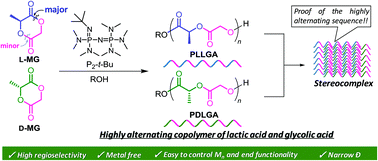
The alternating copolymer of lactic acid and glycolic acid (PLGA) is a highly promising next generation biodegradable material for biomedical and pharmaceutical applications due to its uniform degradation behaviors in addition to its ability to form stereocomplexes. However, versatile synthetic methods toward the narrowly dispersed alternating PLGAs with controlled molecular weights and desired end functionalities have been largely unexplored. Herein, we report the organocatalytic regioselective ring-opening polymerization (ROP) of optically active methylglycolides (L- and D-MGs) to produce highly alternating PLGAs. The ROP of L-MG using a phosphazene base P2-t-Bu/alcohol system in THF at −78 °C achieved the highest regioselectivity (P = 0.95) as well as a good control over the molecular weight (Mn = 6.6–15.6 g mol−1), with a relatively narrow dispersity (Đ = 1.21–1.29). Furthermore, alternating PLGA diol as well as azido-, ethynyl-, and poly(ethylene glycol) monomethyl ether-end-functionalized alternating PLGAs was successfully obtained by employing the corresponding functional alcohol initiators. An enantiomeric 1 : 1 blend of PLGAs prepared from L- and D-MGs formed a stereocomplex with a crystallinity and melting point comparable to the reported data for the stereocomplex from the perfectly alternating PLGAs produced via a polycondensation approach, which implies the high degree of the alternating sequence of the obtained copolymers.


 Please wait while we load your content...
Please wait while we load your content...