Unraveling the gallol-driven assembly mechanism of thermoreversible supramolecular hydrogels inspired by ascidians†
Abstract
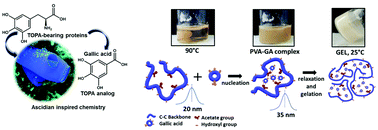
Polyphenols-based supramolecular hydrogels have recently attracted much attention as smart materials for applications in several technologies. Although great advances have been made in this field, there is a challenging need for creating new versatile materials that combine synthesis simplicity and suitable functional properties. In this work, inspired by the hydrogen bonding ability of pyrogallol-bearing proteins found in ascidians, we explored a small gallol analog, gallic acid (GA), as a dynamic crosslinker of poly(vinyl alcohol) (PVA). The fundamentals of the supramolecular assembly mechanism of PVA/GA hydrogels are studied for understanding the final properties of the obtained thermo-reversible hydrogels. The polymer deacetylation degree was a key factor to control the gelation kinetics, morphology, and properties of the supramolecular materials. Furthermore, the intercalation of GA molecules between PVA chains produced polymer crystals with a new spatial arrangement, modifying the elastic modulus of the supramolecular network and increasing its stability in water. With remarkable fast gelation ability, ascidian-inspired PVA–GA hydrogels may provide a promising platform for a wide range of biomedical applications including topical drug delivery of therapeutic proteins, wearable electronic devices, and 3D printing.


 Please wait while we load your content...
Please wait while we load your content...