Vinyl-addition polymerizations of cycloallenes: synthetic access to congeners of cyclic-olefin polymers†
Abstract
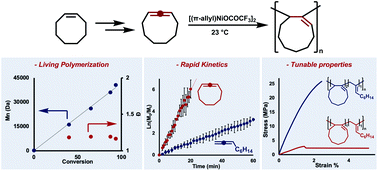
Expanding the scope of cycloolefin-type monomers that polymerize via coordination–insertion mechanisms is an enduring synthetic challenge. Vinyl-addition polymerizations of cyclic olefins are almost exclusively limited to norbornene scaffolds and/or copolymerizations with ethylene. To circumvent these synthetic limitations, we report the vinyl-addition polymerization of cycloallenes using the catalyst, [(π-allyl)NiOCOCF3]2. The polycyclic materials could be accessed in excellent yields (up to 97%), and the polymerization exhibited living character (which was leveraged for copolymerizations with acyclic monomers). Importantly, we found that multiple ring sizes could be incorporated into polymer architectures, and material properties could be tailored by controlling the cycloallene feed ratios.


 Please wait while we load your content...
Please wait while we load your content...