Cationic ether-free poly(bis-alkylimidazolium) ionene blend polybenzimidazole as anion exchange membranes†
Abstract
Two ether-free poly(bis-alkylimidazolium) ionenes with imidazolium cations as part of the main chain were synthesized from 1,4-bis(imidazolyl)butane and 1,4-dibromobutane or 1,4-bis(bromomethyl)benzene via nucleophilic substitution polymerization. Anion exchange membranes (AEMs) were prepared by co-casting with polybenzimidazole (PBI), producing visually homogeneous and mechanically robust blend AEMs. The ion exchange capacity (IEC), water uptake and ion conductivity could be balanced by adjusting the molar ratio of the poly(bis-alkylimidazolium) to PBI polymer in the blend. For example, the blend AEM of PBuIm-37%/PBI prepared from the oligomer derived from 1,4-dibromobutane with an IEC of 1.36 mmol g−1 showed a tensile strength of 5.3 MPa at room temperature and a hydroxide conductivity of 74 mS cm−1 at 80 °C and excellent stability over 500 h in 1 mol L−1 KOH at 60 °C. The present work opens up a new route towards ether-free AEM design and fabrication with a wide potential structural scope.
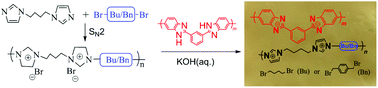


 Please wait while we load your content...
Please wait while we load your content...