Tunable hydantoin and base binary organocatalysts in ring-opening polymerizations†
Abstract
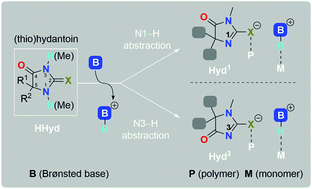
A (thio)hydantoin (HHyd) and organic superbase binary cocatalyst is illustrated as a tunable catalytic tool that enabled the efficient ring-opening polymerization (ROP) of various cyclic ester monomers. A series of designed HHyd molecules with diverse substitutions on the imidazolidine-2,4-dione ring at N1, N3, C5, and C2 allowed the fine tuning of the acidity, steric demand, and nucleophilicity of the (thio)hydantoinate cocatalyst. A partner organic superbase abstracted either the N1–H or N3–H proton of HHyd, leading to a conjugate acid acting as a H-bond donor in electrophilic activations. Commercial Brønsted organic bases, including tertiary amines, amidines, guanidines, and phosphazene, were evaluated for HHyd/base cocatalysis. The amidine DBU and guanidines TMG, TBD, and MTBD were screened as effective bases. Two minimal hydantoins, 1,5,5-trimethylimidazolidine-2,4-dione (HHyd2) and 3,5,5-trimethylimidazolidine-2,4-dione (HHyd3), partnered with DBU showed optimal performances, with near-quantitative conversions and narrow dispersities, in the ROPs of various cyclic ester monomers. HHyd2/DBU obviated the potential for epimerization and/or transesterification in the ROP of L-lactide (LLA). The controlled/living nature of the ROP of trimethylene carbonate (TMC) was validated. ROPs of TMC in solvents at room temperature and in bulk at 90 °C were successful: a short reaction time (6 h vs. 0.5 h), high conversion (92% vs. 97%), and narrow dispersity (1.13 vs. 1.12) were observed (solvent vs. bulk). Homopolymers and diblock copolymers of P(TMC-b-LLA) were prepared. The controlled/living nature of the ROPs was supported through kinetics and chain extension experiments, and MALDI-ToF-MS characterizations. A cooperative activation mechanism was proposed and validated using NMR titrations, in which the hydantoinate activated the chain end and the conjugate acid activated the monomer. The high relative cell viability (>90%) of poly(trimethylene carbonate) samples containing the cocatalyst HHyd2/DBU tested via MTT assays on HaCat cells confirmed the desirable biosafety and biocompatibility.


 Please wait while we load your content...
Please wait while we load your content...