Synthesis of aromatic polyketones by nonstoichiometric Friedel–Crafts polycondensation using AlCl3†
Abstract
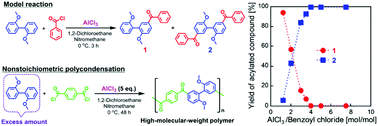
Aromatic polyketones without ether linkages in the main chains were successfully synthesized. Polycondensations were performed between aromatic dicarboxylic acid chlorides and 2,2′-dimethoxybiphenyl (DMB) based on Friedel–Crafts acylation using 5 equivalents of AlCl3 relative to the total amount of monomers. High-molecular-weight polymers were obtained under both stoichiometric and DMB-excess nonstoichiometric conditions. It was found that the equimolar reaction between DMB and benzoyl chloride, using excess AlCl3, selectively yielded a diacylated compound, indicating that the second acylation was much faster than the first. The detailed NMR analysis of DMB and a monoacylated compound revealed that AlCl3 coordinated to the methoxy groups of DMB, and the monoacylated compound with AlCl3 formed a mixture of the compound with AlCl3-coordinated and uncoordinated methoxy groups. The second acylation was determined to be more rapid because the AlCl3-uncoordinated monoacylated compound showed higher reactivity than the AlCl3-coordinated DMB. The change in reactivity before and after the first acylation involved the coordination and dissociation of AlCl3, which plays a key role in the nonstoichiometric polycondensations. The polycondensations presented in this paper are advantageous because high-molecular-weight, rigid, thermally stable, and soluble aromatic polyketones can be obtained without the precise feed control of moisture sensitive dicarboxylic acid chlorides or the use of toxic and expensive superacids.


 Please wait while we load your content...
Please wait while we load your content...