Alkene–azide chemistry: a facile, one-step, solvent- and catalyst-free approach for developing new functional monomers and polymers†
Abstract
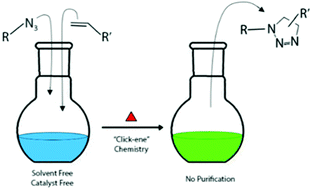
In this study, a one-step Hüisgen 1,3-dipolar cycloaddition reaction, alkene–azide chemistry, was explored using a solvent and catalyst free method forming 1,2,3-triazoline ring-containing molecules. This facile method was utilized by synthesizing a mini library of small molecules, functional monomers, and polymers, which involved mixing a terminal alkene with a terminal azide at elevated temperature. Similar to traditional “click” chemistry, the proposed alkene–azide strategy uses solvent and heavy-metal catalyst free conditions and no further workup or purification steps are required. In our design, small molecule-based compounds, AB-type prepolymers and macromolecules are synthesized at 80–100 °C with more than 90% purity. Polymers made using this chemistry formed high molecular weight macromolecules (Mw = 59.1k, 34.9k, and 49.3k g mol−1). The synthesized new small molecules, functional monomers and polymers were characterized using various chromatographic and spectroscopic methods. The results indicated that the molecular linking, orthogonal green chemistry reaction proceeds well under gentle conditions, forming products with high yields and reserving atom economy. This chemistry provides a facile approach by offering modular synthetic routes, being highly efficient when using equivalent stoichiometric ratios, and by being solvent and catalyst free, which will be of particular interest to the pharmaceutical and industrial settings.


 Please wait while we load your content...
Please wait while we load your content...